BY MARIA BURKE | 17 JANUARY 2024
The finding of significant amounts of metals in aerosols in the stratosphere – likely originating from the launch and re-entry of spacecraft and satellites – could have implications for climate and the ozone layer, Maria Burke reports
There are over 8,000 satellites currently in orbit, according to the Union of Concerned Scientists (UCS). But the burgeoning space industry is planning many more, particularly large systems of many satellites working together (constellations). Years ahead of launch, a nation state must file an application for a proposed satellite system with the International Telecommunication Union (ITU) on behalf of the satellite operator. In autumn 2023, a team from the University of British Columbia, Canada, found that between 2017 and 2022 there were over 1m filings for satellites across more than 300 constellations[1].
‘By treating orbital space as an unlimited resource, humanity is creating serious safety and long-term sustainability challenges to the use of low Earth orbit,’ the UCS researchers wrote. ‘There is urgent need for the ITU and its member states to adopt meaningful controls.’

2010: the STS-131 external fuel tank (ET) begins its relative separation from the Space Shuttle Discovery following launch. Image: NASA
As the space industry grows, concerns are mounting over some of the risks, not only from growing amounts of space junk, but also due to atmospheric pollution. Scientists have long suspected that spacecraft and satellites were changing the stratosphere. Now, researchers from Purdue University and the US National Oceanic and Atmospheric Administration (NOAA) have experimental evidence for these concerns. The team sampled the atmosphere up to 19km above the ground in February and March 2023 using instruments attached to aircraft nose cones to sample the most pristine air. They found that 10% of stratospheric sulphuric acid particles (larger than 120nm in diameter) contained aluminium and other elements from spacecraft re-entry.
In total, they detected over 20 elements present in ratios consistent with alloys used in spacecraft[4]. The mass of lithium, aluminium, copper and lead far exceeded those metals found in natural cosmic dust. Additionally, the team modelled a scenario where 50,000 additional satellites would be in orbit by 2030, estimating that up to half of stratospheric sulphuric acid particles would contain metals from re-entry in the coming decades.
‘Our measurements were not looking for the metals from spacecraft re-entry and we were surprised to find them,’ says Dan Murphy of NAOO. ‘It is quite expected that the spent satellites and rockets produce metal vapours when they re-enter the atmosphere. What wasn’t expected was to find those metals in the stratosphere, which is much lower altitude than where the spacecraft burn up. In hindsight, it is a little less of a surprise: we had previously measured iron and other metals from meteors in the stratosphere and so it isn’t unreasonable to think spacecraft would have the same effect.’
It is ‘remarkable’ that the products of spacecraft re-entry occurring above 50km altitude can be measured with such sensitivity in aerosol particles at less than 19km altitude, says Murphy. Hafnium, for example, is not only detectable but quantifiable. Yet hafnium is a minor alloying element used in only one component (<1% of the mass) of some types of launch vehicles. Its appearance, along with niobium, was not expected in the stratosphere, he adds.
However, the team’s findings fit with work done by Leonard Schulz and Karl-Heinz Glassmeier of the Technische Universität Braunschweig, Germany. Their 2021 paper modelled how much man-made material might end up in the atmosphere compared with natural material originating from asteroids, comets and planets[5]. They found that the amounts were already significant in 2019, the year of study. They estimated that anthropogenic material made up about 2.8%, compared with that from natural sources. In future, their models predicted this could rise to between 13 and 40% depending on satellite numbers. In particular, the anthropogenic injection of aerosols into the atmosphere would increase disproportionately, compared with natural sources, from about 7% in 2019 to 30%-94% in future.
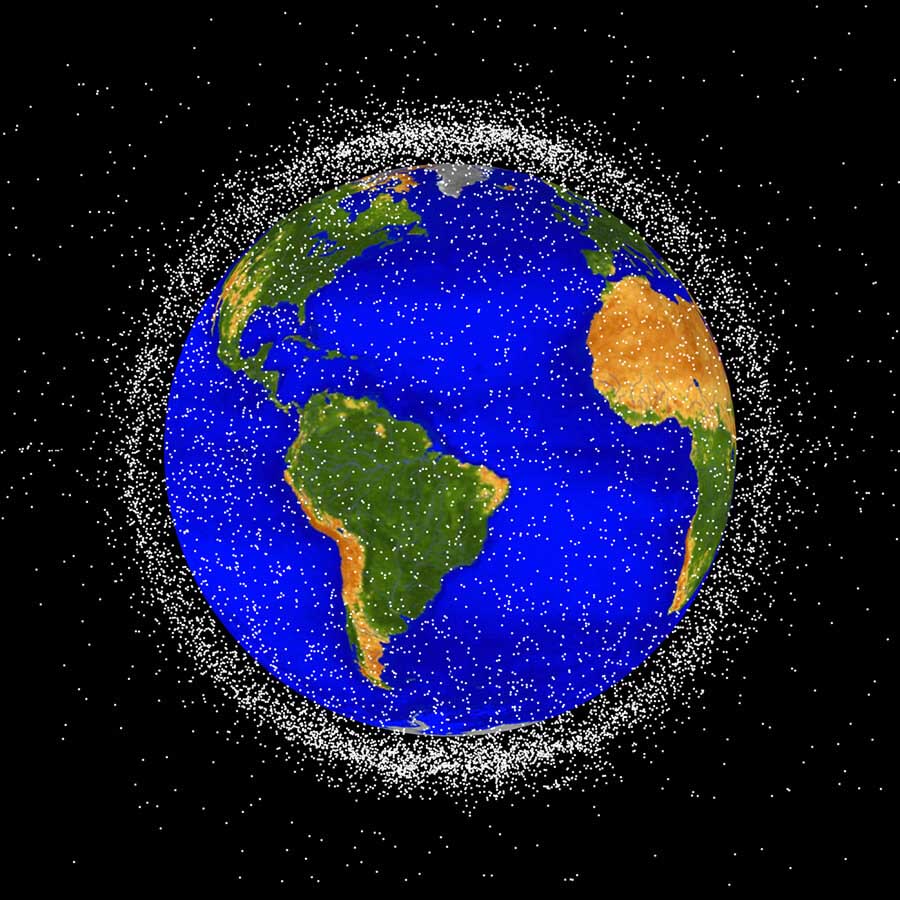
Orbital debris is most concentrated within 2000km of Earth’s surface.NASA
The new observations provide ‘groundbreaking results that confirm our theoretical calculations and modelling,’ says Schulz. ‘[Murphy’s team] also found certain relative metal abundances, which fit well with values we calculated. In general, their observations only strengthen the concerns that our initial assessment raised – the amount of metals injected into Earth’s atmosphere is already significant and, for some elements, is starting to dominate over natural meteoric input. This raises concerns about possibly detrimental environmental effects on the atmosphere of Earth.’
Also in 2021, Aaron Boley and Michael Byers from the University of British Columbia were pointing out the risks of the rapid development of mega-constellations of satellites. One of these risks concerned changes to the Earth’s upper atmosphere[6]. In their models, they suggested that satellite re-entries from the Starlink mega-constellation alone – Elon Musk’s SpaceX venture – could deposit more aluminium into Earth’s upper atmosphere than the amounts originating from meteoroids, meaning they could become the dominant source of high-altitude alumina.
The UBC team calculated that on average almost 2t of metal could be re-entering Earth’s atmosphere every day; Starlink satellites have a dry mass of about 260kg. ‘While small compared with the 54 daily tonnes of meteoroid mass, the satellites are mostly aluminium; most meteoroids, in contrast, contain less than 1% aluminium by mass. Thus, depending on the atmospheric residence time of material from re-entered satellites, each mega-constellation will produce fine particulates that could greatly exceed natural forms of high-altitude atmospheric aluminium deposition, particularly if the full numbers of envisaged satellites are launched,’ the team noted.
They also pointed out that various geoengineering projects have proposed using deposition of aluminium in the atmosphere as a way to alter the fraction of sunlight diffusely reflected by Earth (albedo). These proposals have been scientifically controversial and controlled experiments encountered substantial opposition. However, mega-constellations will begin this process as an uncontrolled experiment, the researchers warned.
The observations of anthropogenic atmospheric metals above Antarctica described in Murphy’s paper are not the first of their kind, Xinzhao Chu of the University of Colorado, Boulder, US, says. ‘In January 2003, our [sensing] system at Rothera Station, Antarctica detected relatively dense sporadic iron layers 112km above Earth’s surface, an altitude at which it’s exceedingly rare to observe such high iron densities[7]. This unusual phenomenon was linked to the Columbia space shuttle launch three days earlier, and thousands of miles away. In our case, the source of the iron was the spacecraft’s engine exhaust as it departed Earth’s atmosphere, but Murphy’s paper concerns material ablated from satellites upon re-entry, which, because these satellites burn up, represents a much larger volume of metals. This result means that the satellite-derived metals that Murphy et al observed may have far greater consequences than the iron traces we detected 20 years ago.’
Potential impacts
Scientists are just starting to think about what the metals might do in the stratosphere, Murphy says. ‘To be clear, the amount of metal is small compared to the amount of sulphuric acid that is naturally in the stratosphere, so we aren’t talking about a massive change like that after the eruption of Mt. Pinatubo. We are talking about whether sulphuric acid particles with metal impurities behave the same as sulphuric acid particles without those impurities. There could be effects on the formation of polar stratospheric clouds or chemistry. We don’t know for sure there are any bad effects. What makes me personally uncomfortable is that we don’t know the answer when the space industry is growing so rapidly.’
At present, the refractory material in stratospheric particles is mostly iron, silicon, and magnesium. If amounts of aluminium (and other elements) increased to comparable levels, there are several possible effects. With a larger variety of metals present, novel stratospheric chemistry or unusual optical properties are possible. For example, aluminium and novel elements could impact the size distribution of the stratospheric aerosol layer. Larger particles coagulate more slowly than smaller particles, so adding man-made material to meteoric smoke could increase the number of particles. This would mean that sulphuric acid particles would be smaller and more plentiful, which would scatter light differently and affect the balance of energy between Earth and the atmosphere.
In Schulz’ view, the biggest concern is the possibility of an enhanced loss of ozone in the ozone layer. ‘Also, there might be influences on mesospheric and stratospheric cloud formation as well as effects on the mesospheric metal chemistry. Even effects on Earth’s climate can’t be ruled out. In my view, we should not wait until we have absolute certainty to take preventive measures but act now as the significance of the human-made injection is already clear.’
What makes this new study so interesting is that the aerosol particles under study were collected from the Antarctic stratosphere, says Chu. ‘I worry about metals such as aluminium, copper and lead because they are not naturally contained in meteoric metals and are therefore foreign to the Antarctic atmosphere and surface. Will these spacecraft metals influence ecological systems in Antarctica and in the Southern Ocean surrounding Antarctica when they reach the surface? Will they change the composition of the thermosphere, mesosphere, stratosphere, and troposphere, which in turn causes changes to the radiation energy budget and the chemical reactions that occur there? Even for iron and magnesium, the two major metal species released by meteoroids, the increased supply of these metals from spacecraft in coming decades could potentially pose new changes.’
‘Changes to the atmosphere can be difficult to study and complex to understand,’ says Dan Cziczo of Purdue University, US, who was also involved in the study with NOAA’s Murphy. ‘But what this research shows us is that the impact of human occupation and human spaceflight on the planet may be significant – perhaps more significant than we have yet imagined.’
Metals and meteoroids
Metals, such as iron, magnesium, sodium, potassium, nickel, calcium and lithium exist naturally in the Earth’s middle and upper atmosphere. They come from meteoroids, small, rocky or metallic bodies that orbit the sun and shed material as they burn up in Earth’s atmosphere. Once in the atmosphere, metal atoms and ions undergo complex chemical reactions and form various metal layers in the thermosphere and mesosphere (the Earth’s atmosphere is separated into various layers with the troposphere nearest Earth, followed by the stratosphere, mesosphere, thermosphere and exosphere)[2]. Such meteoric metal layers have been used as tracers by remote sensing instruments to profile atmospheric temperatures, winds and waves[3].
Eventually, meteoric metals fall through the atmosphere and reach the Earth’s surface. During this sedimentation, metal atoms and ions can form smoke particles and become important components in aerosols in the stratosphere, a layered mass of protective gases surrounding Earth, which sits above the troposphere and below the mesosphere. The aerosol particles that form in the stratosphere help protect and buffer the ozone layer. They consist mainly of sulphuric acid derived from the oxidation of carbonyl sulphide and, after volcanic eruptions, sulphur dioxide. About half of the smaller sized particles (120-600nm in diameter) usually contain small amounts of metals and silicon derived from meteoroids[4].
References
- A. Falle, et al, Science, 2023, 382, 150
- X. Chu, et al, Geophys. Res. Lett., 2020, 47, e2020GL090181
- X. Chu and Z. Yu, JGR Space Physics, 2027, 122, 6812
- D. M. Murphy, et al, PNAS, 2023, 120, e2313374120
- L. Schulz and K-H. Glassmeier, Adv. Space Res., 2021, 67, 1002
- A. C. Boley and M. Byers, Sci. Rep., 2021, 11, 10642
- M.H. Stevens, et al, Geophys. Res. Lett., 2005, 32, L13810